 In the fast-paced world of industrial manufacturing, durability and precision matter. From heavy-duty equipment to complex machinery, every component relies on clear, long-lasting identification. At NFI Corp, we specialize in delivering high-quality labels engineered to withstand the toughest conditions—because in your industry, failure is not an option.
In the fast-paced world of industrial manufacturing, durability and precision matter. From heavy-duty equipment to complex machinery, every component relies on clear, long-lasting identification. At NFI Corp, we specialize in delivering high-quality labels engineered to withstand the toughest conditions—because in your industry, failure is not an option.
Built for Extreme Environments
Our labels are designed to perform where others fail. Whether facing exposure to heat, chemicals, abrasion, or outdoor weather, we offer materials that ensure legibility and durability over time:
- Polycarbonate & Polyester Labels – Tough enough to resist scratching and fading.
- Aluminum – Ideal for harsh environments requiring permanent marking.
- Domed Labels – Offering both style and protection with a resilient, crystal-clear finish.
- Capturite® Self-Laminating Labels – Perfect for variable data applications, ensuring information is secure and protected.
How We Stand Out
 What sets NFI Corp apart isn’t just our material options—it’s our commitment to customization and quality assurance. We don’t offer one-size-fits-all solutions. Instead, we partner with each client to design and manufacture labels tailored to specific applications, ensuring performance and compliance.
What sets NFI Corp apart isn’t just our material options—it’s our commitment to customization and quality assurance. We don’t offer one-size-fits-all solutions. Instead, we partner with each client to design and manufacture labels tailored to specific applications, ensuring performance and compliance.
- Customization: Shapes, adhesives, finishes, and laminates tailored to your needs.
- Expertise: Decades of experience serving industrial leaders.
- Innovation: Advanced printing technologies that deliver precision and consistency.
- Compliance: Labels designed to meet industry regulations and standards.
The NFI Difference
While competitors may offer standard labels, NFI Corp provides solutions that work as hard as you do. Our labels aren’t just identifiers—they’re tools that ensure safety, traceability, and efficiency in your manufacturing process.
When quality matters, trust NFI Corp to deliver.



 Start with the
Start with the  Partner with Experts
Partner with Experts
 At NFI Corp, we understand the critical role labels play in protecting end users and manufacturers alike.
At NFI Corp, we understand the critical role labels play in protecting end users and manufacturers alike. why our team works closely with clients to ensure labels are custom-designed for their exact application—meeting not only functional needs but also aesthetic and branding goals.
why our team works closely with clients to ensure labels are custom-designed for their exact application—meeting not only functional needs but also aesthetic and branding goals.
 At NFI Corp, we design and manufacture
At NFI Corp, we design and manufacture 

 At NFI Corp, we produce nearly all our products in-house — from materials sourcing to engineering to final production — right here in the USA. That means we rely on very few imported components that may be subject to sudden tariffs or international supply chain issues. By keeping our manufacturing under one roof, we maintain full control over quality, timelines, and pricing.
At NFI Corp, we produce nearly all our products in-house — from materials sourcing to engineering to final production — right here in the USA. That means we rely on very few imported components that may be subject to sudden tariffs or international supply chain issues. By keeping our manufacturing under one roof, we maintain full control over quality, timelines, and pricing. off for our customers.
off for our customers.

 At NFI Corp, every solution is custom-designed to meet the unique needs of your facility or product line. We collaborate closely with clients to develop materials and adhesives that meet sterilization and sanitation requirements, and we use advanced printing technologies to deliver crisp, scannable graphics every time.
At NFI Corp, every solution is custom-designed to meet the unique needs of your facility or product line. We collaborate closely with clients to develop materials and adhesives that meet sterilization and sanitation requirements, and we use advanced printing technologies to deliver crisp, scannable graphics every time.





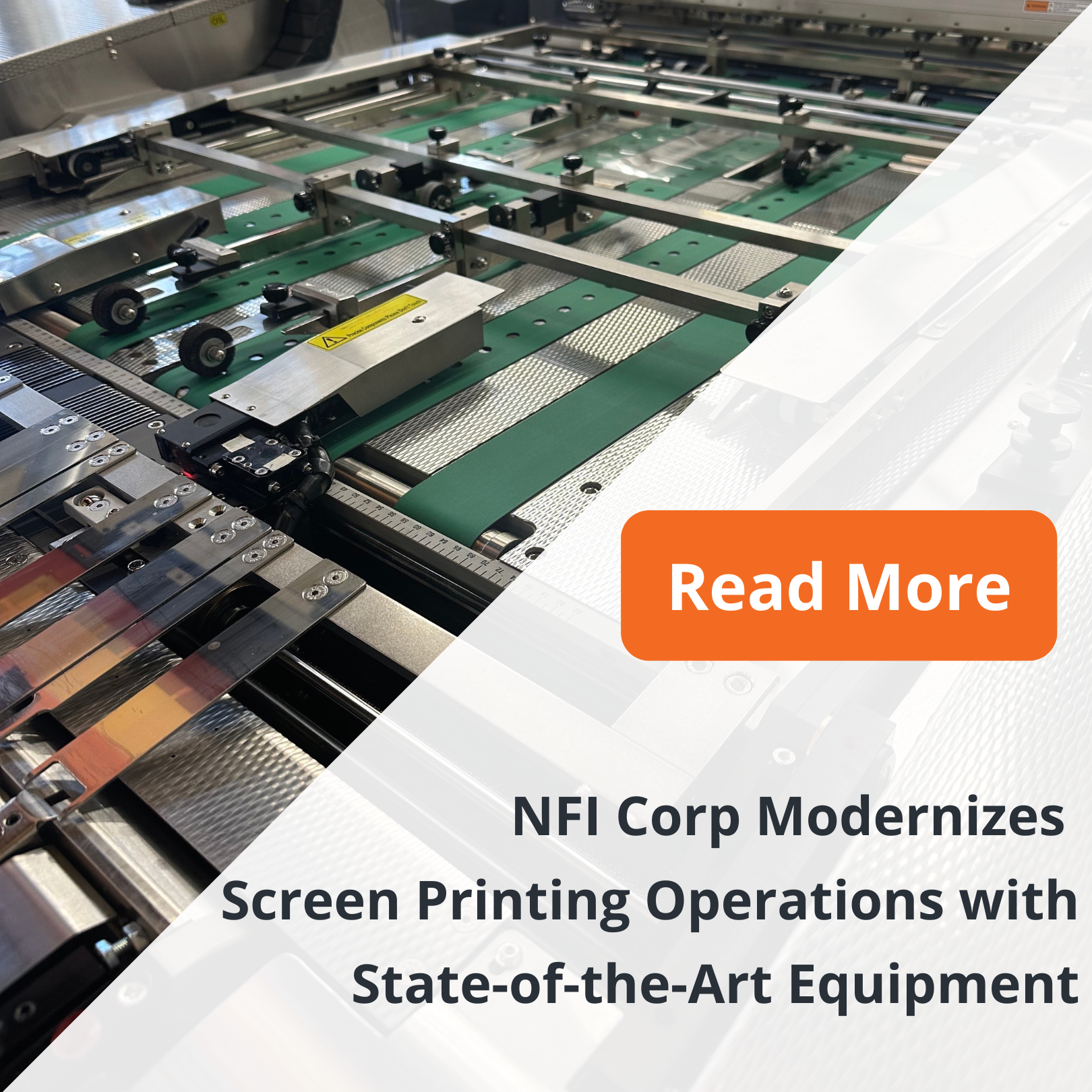
 We have always taken great pride in our
We have always taken great pride in our  At NFI Corp, we’re proud to combine six decades of expertise with the latest technology to deliver industry-leading solutions. This investment is just one example of how we’re committed to staying at the forefront of
At NFI Corp, we’re proud to combine six decades of expertise with the latest technology to deliver industry-leading solutions. This investment is just one example of how we’re committed to staying at the forefront of 

